Understanding Dalton's Law in diving.
Dalton's law (also called Dalton's law of partial pressures) states that "The total pressure exerted by a mixture of gases is equal to the sum of the pressures of each of the different gases making up the mixture – each gas acting as if it alone were present and occupied the total volume."
Well that's all good but what does it mean? SCUBA divers can be obsessed with gas laws, you probably remember learning "Boyes Law" (pressure and volume) during your open water program?
So it's time to look at another gas law being "Daltons law". It could be said that Daltons law or more specifically "Daltons triangle" might be the holy grail of dive theory? So pour yourself a nice cup of tea, grab a comfy chair, read on and you decide for yourself.
John Dalton (the man)
John Dalton was an English Chemist born 1766 - 1844. He is best known for his atomic theory, but he is also renowned for his research into color-blindness that both he and his brother suffered from. He formulated the theory of partial pressure in 1801 and its applications to diving are wide and extremely important, especially in technical and enriched air nitrox diving.
While Archimedes Principle, Charles Law and Boyles Law focus on the physical effect, Henrys Law and Daltons Law explain the physiological effect.
Daltons Law of partial pressure enables us to predict how much of a specific gas is dissolved into the blood at a given depth. His law very much relates directly to physiology and describes behaviours of any gas in a mixture of gases. This is important because of the physiological effect each individual gas can have on a diver when the partial pressure rises above a particular level.
At higher partial pressures, gases in the blood alter the electrical properties in the cerebral cell membranes and may cause narcotic anaesthetic, nitrogen narcosis or toxicity in the body.
Understanding this law of partial pressure helps determine air supply, gas volumes, maximum depth when diving with enriched air; it helps us plan our dives, determine the ideal gas mixture for the type of diving we wish to do and when to change gases underwater (for technical decompression diving). It also helps us avoid problems (CNS/Pulmonary toxicity) with individual gases under pressure within a mixture.
Both Henry`s Law and Dalton`s Law are used to determine decompression models used in dive tables and computers
Gas toxicity when diving
Nitrogen, oxygen, and carbon monoxide can all have toxic or narcotic effects during diving. Nitrogen Narcosis occurs when the partial pressure of nitrogen (PN2) is to 2.37 - 3.16 bar (20 to 30 + m) and above of absolute breathing air and is caused by the partial pressure of nitrogen in the blood.
Oxygen toxicity happens when partial pressures of oxygen exceed 1.4 - 1.6 bar and above
(56-67 + msw). More of a concern when technical diving or using enriched air nitrox (EANx) than when diving with air within recreational limits. Small amounts of carbon monoxide in your breathing air that are harmless at the surface can become toxic at depth, causing carbon monoxide poisoning. This potential danger of carbon monoxide poisoning can be pretty much be eliminated by getting your cylinders filled at a reputable, clean and well maintained fill station.
High pressure nervous syndrome can occur when divers are deeper than 150-180 meters. Especially when the dive is rapid, and the diver is breathing helium and oxygen mixes. Like Nitrogen narcosis, it resolves itself when the diver ascends, or the rate of descent is slowed. It can be avoided by adding a small amount of a narcotic gas, like nitrogen, to the mix.
Boyles Law explains that when breathing air at depth, each breath contains more molecules than at the surface (density directly proportional to pressure). This means that at depth, we are breathing the same percentage of each gas in a mixture as we would at the surface but the number of molecules we breathe increase with pressure. From this we can show that - as the pressure increases, the Partial Pressure (NOT THE PERCENTAGE, THE PERCENTAGE NEVER CHANGES) will increase.
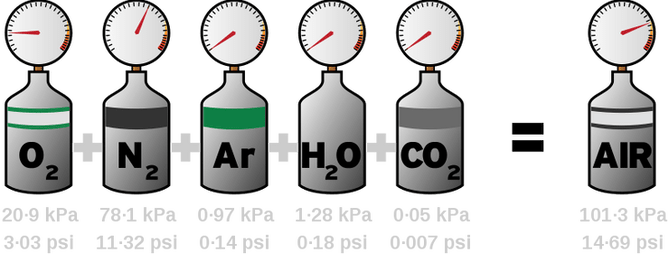
e.g., 21% Oxygen + 79% Nitrogen =100% Air is the same at every depth.
The physiological effect of a gas changes as partial pressure changes, as does the surface equivalent, but the actual percentage remains the same.
Each gas within a mixture exerts its own individual pressure, acting as if it alone were present and occupied the total volume. This is called partial pressure (abbreviated "pp" or "P" before a gas such as "PO2" for "partial pressure of oxygen") as it is part of the total pressure exerted by that gas mixture and is proportional to the number of molecules of that particular gas within the gas mixture. Instead of counting molecules, Dalton’s Law says that we can use partial pressures.
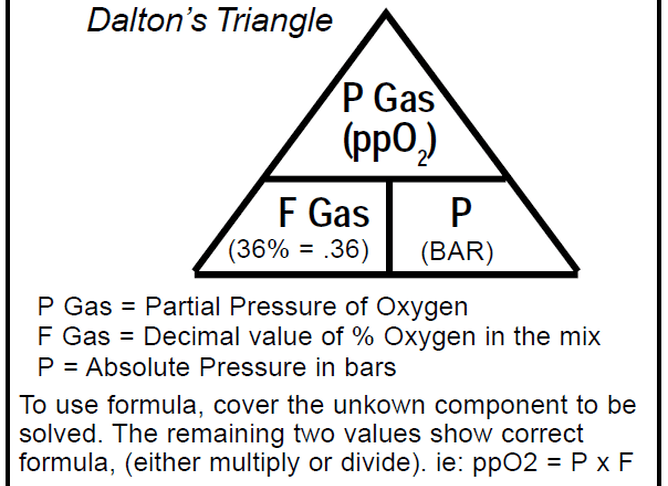
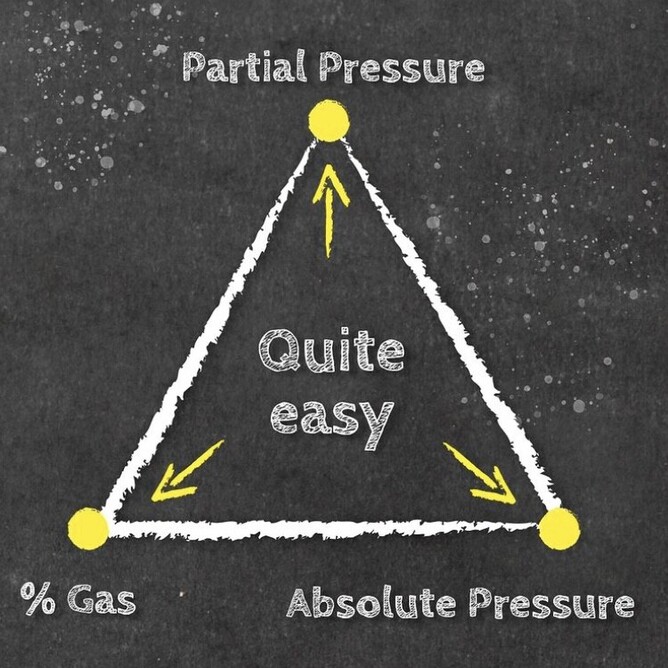
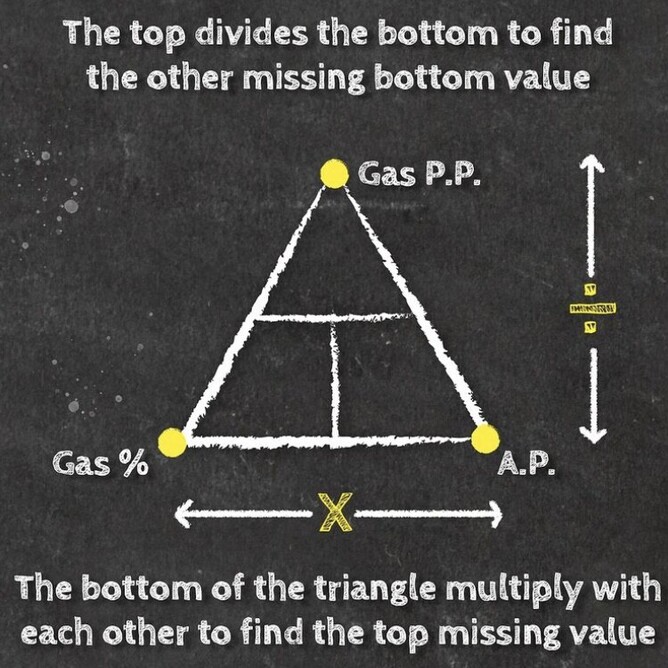
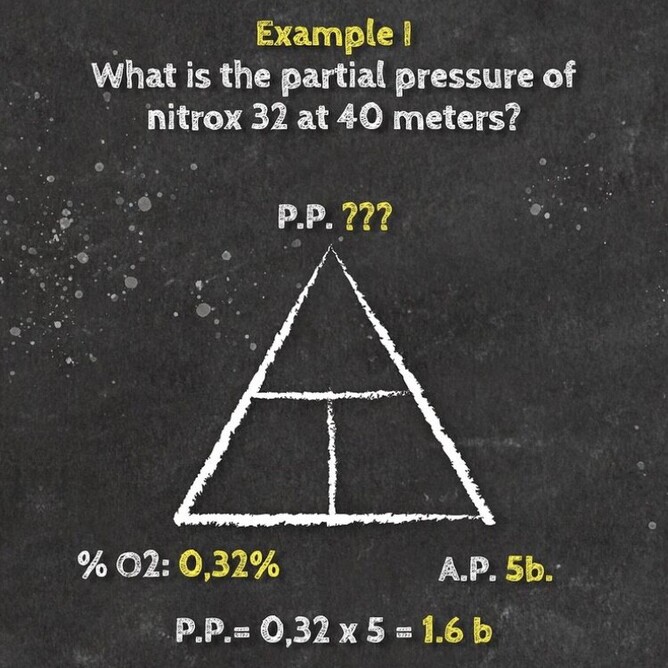
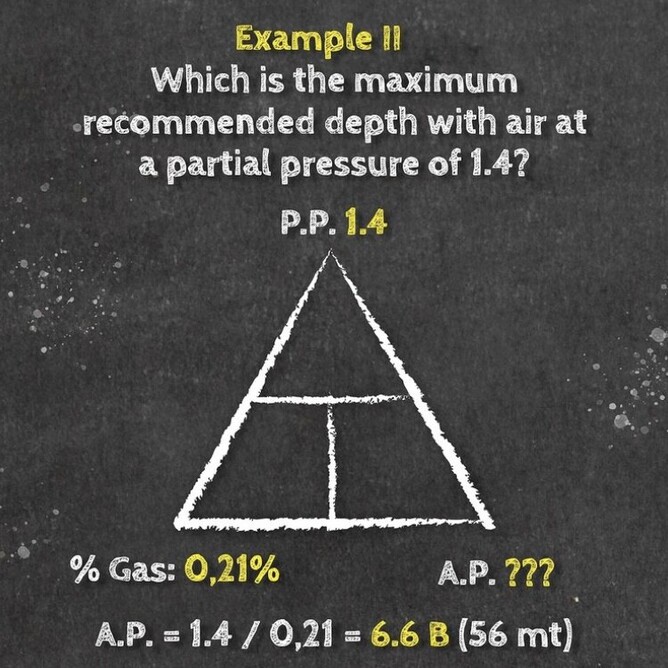
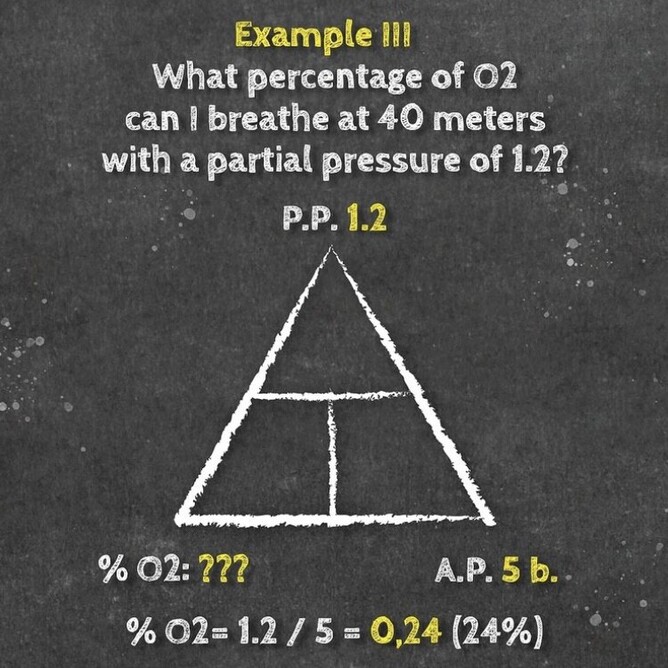
If you take a gas underwater, the pressure increases with depth. The partial pressure of each gas in the mix also increases proportional to its fraction in the mix. You can determine the partial pressure by simply multiplying the gas percentage (in decimal) in the mix by the total absolute pressure.
Daltons triangle is a simple equation that has many uses for both enriched air nitrox divers and technical divers alike. Once you understand how the formally works you can calculate amongst other things:
- Maximum operation depth of a gas
- Partial pressure of a gas at depth
- Optimum mix for a specific dive depth
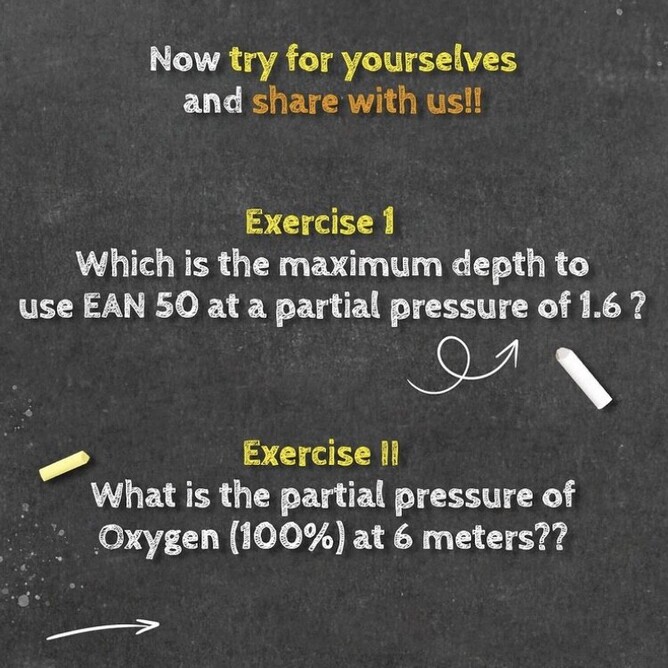
Below is our idiots guide to how to use Daltons Triangle and remember if you want to learn more about Daltons law of partial pressure then maybe sign up for a Science Of Diving program or Enriched Air Nitrox program, CLICK HERE FOR MORE DETAILS.
Learn more about dive theory and get more from your diving, take a look HERE at the SSI Science Of Diving program and sign up today!